Otto Heinrich Warburg was born October 8, 1883 in Freiburg, Baden-Württemberg, Germany. At that time, his father (Emil Warburg) was an eminent professor in physics at the University of Freiburg. Otto’s mother was Elizabeth Gaertner. She is spoken of as a woman of great vitality and wit - Otto thought that essential traits of his personality stemmed from his mother and her side of the family. Though Otto was born into a renowned Jewish family, his father had converted to Christianity before his birth and his mother was a born Protestant. The family lineage originated in the beautiful little town of Warburg, about thirty miles west of Göttingen, in the mid-16th century.
Otto, along with his three sisters, grew up in an academic environment. As was common among professors’ families, the Warburgs resided at Emil’s institute (to allow him to concentrate on research). Otto’s father worked incessantly.
In 1896 (when Otto was 12), the family moved to Berlin, where Emil had become Director of the Institute of Physics at the University of Berlin. Through his membership in the prestigious Prussian Academy of Sciences, Emil was in close contact with scientific colleagues such as Albert Einstein and Max Planck. The Warburg home became a stimulating cultural center which brought young Otto into close contact with leading Berlin academics as well as with artists.
Truth is more likely to come out of error if it is clear and definite, than out of confusion, and my experience teaches me that it is better to hold an understood and intelligible opinion, even if it should turn out to be wrong, than to be content with a muddle-headed mixture of conflicting views, sometimes called impartiality, and often no better than no opinion at all.
- Otto Heinrich Warburg


Otto Warburg's father was also an accomplished pianist - during social evenings, it wasn’t uncommon to see Einstein playing the violin & Emile Warburg and Max Planck on the piano - along with other colleagues such as J.H. van’t Hoff and Walter Nernst contributing to the musical, literary, and philosophical entertainment.
At the age of 18, Otto decided to study chemistry. He left Berlin in 1901 to enroll at the University of Freiburg, so that he could study with the renowned Dr. Emil Fischer.
Dr. Fischer received the Nobel Prize in 1902 for the synthesis of sugars and purines. He was a demanding teacher, requiring precision in experiments and thinking, and perseverance in science. Otto’s early research with Dr. Fischer focused on polypeptides. In 1906, Dr. Warburg completed his Ph.D. dissertation chemistry, entitled Über Derivative des Glycocolls, Alanins und Leucins. Über die 1-Brompropionsäure und das 1-Alanylglycin (On derivatives of glycine, alanine and leucine. On 1-bromopropionic acid and alanylglycine).
At the University of Freibur,g Dr. Warburg became acquainted with Julian Huxley (an evolutionary biologist), Viktor von Weizsäcker (a founder of psychosomatic medicine), Archibald Vivian Hill (an English physiologist and later Nobel Prize winner in physiology), and Otto Meyerhof (a biochemist and Nobel Prize winner in physiology). These great minds had a lasting and significant influence on young Dr. Warburg.
Dr. Warburg wanted to apply the concepts and methods he had acquired in his study of chemistry and physics to understand the energetic processes of life. He started experimenting with different types of cells: bacteria, yeast, and red blood cells. During the summers of 1908 to 1911, often along with Meyerhof, he visited the Marine Station in Naples, Italy where he made his first astute observations with sea urchin eggs. In these embryological investigations of sea urchin eggs, he had observed a rapid increase in O2 uptake and subsequent rapid cell division upon fertilization. He postulated that cancer tissues of rapid cellular division might also uptake more O2 than normal tissue.
This work eventually converged into a second doctoral thesis in 1911 on the oxidation of live cells performed with sea urchin eggs as a model system, setting the groundwork for his later investigations into cancer. In the 1920s, Dr. Warburg set out to test whether cancer cells have increased oxygen consumption.
With this firm scientific foundation, Otto was well on his way to a promising career. He was exceptionally ambitious - and confessed he wanted to surpass his father’s own achievements. Keep in mind: by the age of 30, Emil Warburg had reached one of the top positions available to physicists in Imperial Germany (Chair of Physics at the University of Freiburg).
University of Freiburg

Freiburg ca. 1900
Otto still wanted to know more about the biological processes in life, so he moved to the University of Heidelberg to study medicine with Ludolf Krehl. From 1907 until 1932, Krehl was a professor and director of the medical clinic at the University of Heidelberg. Here Otto worked on the process of oxidation. His special interest in the investigation of vital processes by physical and chemical methods led to attempts to relate these processes to phenomena of the inorganic world. His methods involved detailed studies on the assimilation of carbon dioxide in plants, the metabolism of tumors, and the chemical constituents of the oxygen transferring respiratory ferment. During his 8 years at Heidelberg, he published 30 major papers.
In 1911, Otto obtained his Doctor of Medicine degree from the University of Heidelberg. In 1913, he returned to Berlin, where the Kaiser Wilhelm Institute had been founded (and where his previous mentor Emil Fischer was Vice President).

University of Heidelberg Main Building - Constructed 1905
To perform the technical and exacting measurements with oxygen necessary for his experiments in fermentation, Dr. Warburg obtained a manometer. A manometer is simply a device that measures pressures. Having seen the Haldane-Barcroft “blood gas manometer” on a brief visit to Joseph Barcroft’s laboratory at Cambridge, Dr. Warburg began using this instrument. This differed from earlier manometers in that a special device kept the volume of the gas-space constant so that the pressure was the only variable when a gas was formed or removed at constant temperature. Dr. Warburg ingeniously adapted it for the measurement of rates of gas exchanges. Thus, a special advantage of the Warburg manometric technique over other manometric methods was that that it measured increments directly whilst the other analytic procedures measured differences. Hence, the presence of any amounts of O2 or CO2 did not affect the accuracy of the measurements.
Manometry became a key factor in the discovery of the lactic acid fermentation of cancer tissue, in much of the work on cell respiration, fermentation and photosynthesis, as well as in the identification of the iron porphyrin structure of the oxygen-transferring enzyme of cell respiration. Warburg’s manometer was also used to measure CO2 emission, which is equivalent to lactic acid production, from bicarbonate-containing buffers. These contributions were pivotal to his research on metabolism and cancer physiology, which are described in a collection of his early works.
In the 1940s, some aspects of manometry began to be superseded by spectrophotometric techniques, but this was again the result of Dr. Warburg’s fundamental discoveries and methodological ingenuity. It was Dr. Warburg who developed from 1928 onwards the use of the photoelectric cell and the provision of suitable sources of monochromatic light, in the first instance for his measurement of the action spectrum of the “O2-transferring enzyme of respiration”. Much later, in the 1940s, these principles were incorporated into the commercial spectrophotometers, the first to be marketed being that of the Beckman company.
The Warburg Manometer

At the outbreak of the First World War in 1914, Dr. Warburg felt a traditional and national obligation to serve his country, and volunteered (much to the dismay of his mother). He joined the 2nd Regiment Ulanen (3rd Squadron), an elite cavalry unit, first serving as a physician and later as aide-de-camp at the headquarters of the 202nd Infantry Division. He served in France and at the Eastern Front in present-day Estonia and Lithuania. Dr. Warburg was wounded in 1917, possibly during the battle for Riga, and was awarded the Iron Cross First Class.
World War I

The Iron Cross is a former military decoration in the Kingdom of Prussia, and later in the German Empire and Nazi Germany.
After he was wounded, Dr. Warburg’s mother insisted her son could better serve his country not by returning to the battlefield, but rather by returning to academic science. So she wrote to their friend Albert Einstein, requesting he use his influence to convince her son. Einstein complied; Dr. Warburg sought release from active service, which was approved in the summer of 1918.
Dr. Warburg returned to Berlin - and to a lab bench in the Kaiser Wilhelm Institute. As a principal investigator at the new Institute of Biology, he was free to choose his research topic. As funding was limited under the Weimar Republic, the fact that his one-sentence research proposal received full funding speaks to Dr. Warburg’s scientific reputation.
Within a few years of his return from World War I, three main lines of investigation emerged, occupying Dr. Warburg from 1919 until his death in 1970: photosynthesis, cancer, and the chemical nature of the enzymes responsible for biological energy transformations (i.e. oxidations and reductions).In all three, he greatly advanced the methodologies and made fundamental discoveries.

Application to the Notgemeinschaft der Deutschen Wissenschaft (Emergency Association of German Science, the forerunner of the Deutsche Forschungsgemeinschaft). The application consisted of a single sentence, “I require 10,000 marks” and was fully funded.
In 1923, Otto Warburg and Seigo Minami published the first observations on changes in the metabolism of tumors. They had observed that tumors acidified the Ringer solution (an isotonic salt solution, with 2.4 mM NaHCO3) when 13 mM glucose was added, as indicated by a change in the color of organic pH-indicators. In this acidified solution, lactic acid was chemically identified. To better quantify this phenomenon, Otto Warburg modified the Barcroft manometer to measure slices of a Flexner-Jobling rat hepatoma, which he had received from Rhoda Erdmann at the Rockefeller Institute. The amount of lactate produced was calculated from the increase in CO2-formation during a 30-min incubation period. Surprisingly, the tumor tissue had a 70-fold higher rate of lactate formation than the normal liver as well as kidney and heart tissue likewise tested.
This is the observation that would more than 50 years later be referred to as the Warburg Effect. He discovered that lactate production from glycolysis did not depend on the presence of oxygen. That had not been expected, since according to Pasteur, the presence of oxygen should have suppressed glycolysis. The fact that there appeared to be no direct relationship between respiration and glycolysis led to the conclusion that in cancer cells, glycolysis was a reaction which could produce energy, independent of respiration (oxygen consumption). In other experiments with varying glucose and bicarbonate concentrations, it was shown that there was no generalizable difference in oxygen consumption between the tumor and the respective normal epithelial tissue.
In 1924, Dr. Warburg hypothesized that there was a defect in the relationship between glycolysis and respiration. Even though this observation was corroborated with other tumors by several contemporary scientists, the observation that oxygen could not suppress glycolysis prompted him to propose that a damage in respiration leads to carcinogenesis. This came to be a highly controversial issue climaxing in his famous papers in Science in 1956.
Testing the effects of other parameters, Dr. Warburg and coworkers changed the pH of the Ringer solution ranging from pH 7.83 to 6.66 using 1–15 % CO2-N2 gas mixtures, respectively. The rate of CO2-production (interpreted as glycolysis) increased with increasing alkaline pH. Moreover, a tenfold increase in bicarbonate concentration at a defined pH of 7.5 also increased CO2 production. Warburg interpreted these conditions as being like those in blood passing through capillaries, leading at the same time to a modest acidification and to an increase in bicarbonate concentration. In the balance, glycolysis of the tissues would not change. On the other hand, other studies showed that in tissue homogenates, alkalinity increased with dedifferentiation and necrosis of tumors, suggesting that the tumor itself may have a different pH. However, the influence of pH on the growth of tumor cells appeared never to be of interest to Dr. Warburg, despite his interest in hydrogen-transferring systems such as the coenzymes NAPDH and NADH, which led to the characterization of the activity of most glycolytic enzymes in later years.
First Observations on Tumor Metabolism
First Nobel Prize Nomination
Dr. Warburg was nominated for the Nobel Prize in 1926, but the committee decided to award it to Johannes Fibiger for his findings on a gastric tissue growth condition believed to be a cancer induced by a nematode (spiroptera carcinoma) - findings which later turned out to be false.
Iron and Copper Studies
In 1927, Dr. Warburg designed a method of determining trace quantities (10 – 4 mg) of iron (Fe) and copper (Cu) based on the catalytic effects of these ions on the oxidation of cysteine by molecular oxygen. With these methods he established the basic facts concerning the occurrence in biological material of free or “loosely bound” iron and copper (i.e. Fe and Cu which readily chelate with cysteine). He discovered that Fe and Cu are regularly present in human and animal blood plasma and that their concentrations under normal conditions are as constant as are other blood constituents. He established the normal range (which for both Cu and Fe is of the order of 1 mg/liter) and found that the concentration of copper is raised in pregnancy, infections and iron deficiency anemias and that the Fe content falls in iron deficiency anemias. This work was the starting point for the clinical application of Fe and Cu determination in human serum or plasma, a matter of diagnostic importance especially in Wilson’s disease where the Cu content is greatly decreased.
Fermentation is the metabolic process by which energy is produced in the absence of O2 through the oxidation of organic compounds, typically sugars, to simpler organic compounds, such as pyruvate. Pyruvate is further processed to ethanol by alcoholic fermentation or lactic acid by lactate fermentation.
Dr. Warburg was interested in the chemical basis for the “respiratory ferment” responsible for oxygen transfer in cells. He had already postulated in 1914 that iron had a catalytic function in cellular respiration. Visiting Warburg’s lab, Alan Hill brought to his attention that the inhibition of respiration by CO was light-sensitive.
Thus, Dr. Warburg discovered that inhibition of respiration by carbon monoxide (CO) can be reversed by light. In studying the O2 consumption by yeast cells in the presence of CO in the gas phase, he developed a method based on the photochemical recovery of respiration by dissociation of CO bound to the respiratory enzyme. Photosensitivity of the adduct between hemoglobin and CO had been demonstrated years before by Haldane and Lorrain-Smith.
The effect of light, which allowed Dr. Warburg to unveil that heme is the prosthetic group of the respiratory enzyme, is due to the much higher quantum yield of photodissociation of CO as compared to O2 bound to reduced heme proteins. By measuring the rate of respiration recovery as a function of the wavelength of the irradiating light in the presence of variable mixtures of O2 and CO, Warburg and Negelein determined the photochemical action spectrum of the respiratory enzyme - cytochrome-c-oxidase. It is remarkable to see the spectrum of cytochrome-c-oxidase determined so many years ago, so accurately.
Today, we know that there are indeed five proteins with iron for the electron transport and that cytochrome-c-oxidase oxidase is part of complex IV. Dr. Warburg furthermore postulated the respiratory proteins to be localized in the “grana” of cells, which years later were identified as mitochondria. In a paper published in 1928, The Chemical Constitution of Respiration Ferment, he described the essential “ferments” involved in cell respiration.
The Respiratory Ferment and Cytochrome-c-oxidase
Kaiser Wilhelm Institute and The Nobel Prize

Otto Warburg in his lab at the Kaiser Wilhelm Institute for Biology in Berlin-Dahlem in 1931. Bundesarchiv (German Federal Archives) Bild 102–12525, photographer unknown.
Dr. Warburg loathed academic tourism and believed it to be a waste of time. But he did make two visits to the USA in the 1920s, visits which had profound consequences on his career. On the first occasion in 1924, he visited Jacob Loeb, a friend of the Warburg family, at the Rockefeller Institute in New York. He also gave talks at other universities. In 1929, he was a guest in the laboratory of Barron at Johns Hopkins Medical School, where he performed experiments on the enzymatic nature of chemical reductions in blood cells.
On this visit, with the support of Jacob Loeb, he also negotiated with the Rockefeller Foundation with regard to establishing research institutes in Germany. He suggested the building of a small institute for cell physiology, as well as a larger institute for physics under the direction of Max von Laue. He envisioned collaborating with physicists especially in the field of radiation (the application of radiation to biochemical problems had been a major source of his recent successes).
Within six months, the Rockefeller Foundation provided 635,000 marks for the purchase of the land required for the two institutes, 600,000 marks for the building and equipment of the institute for cell physiology, and about 1.5 million marks for an institute of physics, all under the wing of the Kaiser Wilhelm Gesellschaft. The Institute of Cell Physiology was inaugurated in December 1931, and Otto Warburg served as its sole director.
Dr. Warburg further investigated the metabolism of tumors and the respiration of cells, and in 1931 was finally awarded the Nobel Prize in Physiology for unraveling the oxygen-transferring ferment of respiration and for his discovery of the nature and mode of action of the respiratory enzyme - cytochrome C oxidase (CcO). The award came after receiving 46 nominations over a period of nine years beginning in 1923, thirteen of which were submitted in 1931, the year he won the prize. His father had missed this event by only a few months, having died in July.
In the mid- to late 1930s, some aspects of manometry that Dr. Warburg used in his research began to be replaced by spectrophotometric techniques. Together with the physicist Manfred von Ardenne, Dr. Warburg improved the sensitivity of the spectrometer, which allowed him to measure the light absorbance spectra of pyridine nucleotides. The discovery of differences in the spectral lines with the state of hydrogenation opened new possibilities to measure and determine the activity of enzyme reactions. This involved the transfer of molecular hydrogens from or to these pyridine nucleotides.
Over the next 10 years, using this spectrographic assay, Dr. Warburg was the first to crystalize and characterize 9 of the 13 glycolytic enzymes now known by the reactions they catalyzed. In the now accepted nomenclature these glycolytic enzymes were lactic dehydrogenase, enolase, aldolase, glyceraldehyde-phosphate dehydrogenase, 3-phosphoglycerate kinase, alcohol dehydrogenase, pyruvate kinase, a-glycerophosphate dehydrogenase, and triose phosphate isomerase. These are all key enzymes currently being studied in cancer metabolism. Further technical improvements on the photometer in cooperation with California-based Beckman Instruments made the device commercially available, and this optical test became a world-wide enzymatic and analytical tool.

Nobel Prize in Physiology - 1931
When the National Socialist German Workers’ Party (or Nazi Party) came to power in the early 1930s, people of Jewish descent were forced from their professional positions. Under the Reichsbürgergesetz (Reich Citizenship Act of 1935), the Nazis considered Dr. Warburg a Halbjude (half-Jew). (His mother was Protestant; his father, who had converted to Protestantism, was of Jewish heritage.) Although banned from teaching, Dr. Warburg was permitted to carry on his research.
Most of the extended Warburg family escaped Nazi persecution. Dr. Betty Warburg and her mother Gerta, however, perished in the Sobibor camp in the Lublin district of Poland. A cousin, Helen, perished in Auschwitz, and another cousin, Maria, never left the Brandenburg Euthanasia Center. Dr. Warburg’s three sisters survived by marrying members of Germany’s high society and converting to Christianity.
In 1941, Dr. Warburg lost his post briefly when he made critical remarks about the Nazi regime. Throughout his life he generally remained apolitical, focusing instead on his lab experiments and writings.
A few weeks after losing his post, a personal order from Hitler's Chancellery ordered him to resume work on his cancer research (apparently his research was too valuable to lose). In 1942, he was appointed to a national committee entrusted with fighting cancer, a disease that Hitler morbidly feared.
In 1943, when air attacks made life in Berlin dangerous, Dr. Warburg, along with his staff and equipment, moved about 30 miles northwest to the Liebenberg estate - which Prince Eulenberg, a Prussian nobleman, had placed at his disposal. Here his work continued undisturbed until 1945, when the advancing Russias occupied the area and confiscated all his lab equipment. Although no one has ever discovered who was responsible for this action, Russian Commander-in-Chief, Marshall Zhukov, subsequently invited Dr. Warburg to see him and told him, in the name of the Russian Government, that the dismantling of his laboratory had been in error. Orders were issued to return his apparatus and books, but, alas, they could not be traced.
In 1944, Dr. Warburg was nominated for a second Nobel Prize in Physiology by Albert Szent-Györgyi, for his work on nicotinamide, the mechanism and enzymes involved in fermentation, and the discovery of flavin (in yellow enzymes). According to some sources, he was awarded this second Nobel, but was unable to accept it owing to a 1937 decree by Hitler preventing Germans from accepting the award. Regardless, Dr. Warburg continued his tline of research into the “hydrogen transfer of cell enzymes” and even attracted a third Nobel nomination that was highly politicized.
Unfortunately, much of his research was interrupted during the final war years.
Throughout the 1940s, Dr. Warburg practiced his own recommendations in maintaining a disciplined lifestyle. He grew his own vegetables, drew water from an unpolluted well, had his bread baked with grains from wheat not treated with pesticides, and kept his own poultry. He also remained active doing sports, taking long walks and horseback riding. After his favorite sister Lotte died of cancer in 1948, Dr. Warburg quit smoking.
World War II

After the war, it took some years until Dr. Warburg could return to acceptable working conditions. He also came under investigation for his role in Nazi Germany. Although the headquarters of the German armed forces classified Warburg’s institute as crucial to the war effort, Dr. Warburg later refuted that he had ever performed war-related research. He was eventually readmitted into the international scientific community.
Post War Years, Inventions and Accolades
Otto Warburg with German and American colleagues at the Woods Hole Oceanographic Institution, USA in 1949. Left to right: Saul Korey, David Nachmansohn, Dean Burk, Albert Szent-Györgyi, Otto Warburg, Otto Meyerhof, Carl Neuberg and George Wald.


Some of Dr. Warburg’s work, although aimed solely at gaining knowledge of the laws of nature, had other far-reaching practical and economic consequences. His discovery of nicotinamide as a cell constituent in 1935 led Conrad Elvehjem to its identification as the anti-pellagra vitamin, in preventing and curing pellagra. Later, the inhibitory effects of nicotinamide on the mycobacteria responsible for leprosy and tuberculosis led to the discovery that its chemical cousin isonicotinic acid hydrazide (isoniazid) is even more effective than nicotinamide. This drug is now widely used to control tuberculosis.
By 1952, with numerous accolades accorded him, he was often invited to lecture in Europe and the USA. Also, despite international censure during the war, his membership in the Royal Society (he became a member in 1934) was never rescinded. In fact, Dr. Warburg received an honorary doctorate from Oxford University. He was finally elected as a member of the Academy of Sciences (reestablished in East Berlin), joining the ranks of his father, Albert Einstein and Max Planck.
During the final period of his life, Dr. Warburg continued working on both photosynthesis and tumor metabolism, particularly on the role of alterations of the glycolysis cycle in tumorigenesis. He continually sought means to improve quantification in biological research and wrote prolifically. His publications were groundbreaking, because Dr. Warburg used quantitative physical and chemical approaches to investigate the rapid growth of cancer cells.
In the post-war years, Dr. Warburg received job offers from several different countries (including Russia and the US) and from several German cities.
Eventually, in 1948, he traveled to the USA, accepting invitations from Dr. Robert Emerson, a botany professor at the University of Illinois, and Dr. Dean Burk at the Cancer Institute in Bethesda to perform photosynthesis experiments in their laboratories. Returning to Berlin in 1950, he worked at the Kaiser Wilhelm Institute (which in 1952 was converted into a Max Planck Institute), where he continued working until his death in 1970.
Following up on his earlier work, in the 1950s and 60s, Dr. Warburg studied how the aerobic glycolysis of cancer cells may arise. He found that fibroblasts in tissue culture develop into fibrosarcoma cells when repeatedly exposed for short periods to low oxygen pressure. Dr. Warburg showed that embryonic mouse cells acquire the metabolic characteristics of cancer cells in tissue culture at low oxygen pressure within 48 hours (i.e. in the course of two cell divisions). Most importantly, if normal oxygen pressure is restored, the cancer metabolism remains and supports growth.
Dr. Warburg continued to assert his hypothesis on the cause of cancer, claiming that “the respiration of all cancer cells is damaged”. His famous paper published in 1956 in Science, entitled On the origin of cancer cells, set off years of controversial debate. Many researchers argued that Warburg's findings really identified the effects (and not the causes) of cancer since no mitochondrial defects could be found that were consistently associated with malignant transformation in cancers (at that time).
Eventually, his theory that cancer starts from irreversible injury to cellular respiration eventually fell out of favor amid research pointing to genomic mutations as the cause of uncontrolled cell growth.
Summarized in a few words, according to Dr. Warburg, the primary cause of cancer is the replacement of the respiration of oxygen in normal body cells by a fermentation of sugar. All normal body cells meet their energy needs by respiration of oxygen, whereas cancer cells meet their energy needs in great part by fermentation.
He presented these concepts at prestigious meetings, one being the Annual Meeting of the Nobel Laureates in 1966 in Lindau, an island on Lake Constance (Germany). Here he still faced much scientific dissent. His idea that what allowed for the proliferation of cancer cells was energetics at the respiratory level flew in the face of more recent discoveries that focused on the altered genetics of cancer cells leading to tumor growth.
The respiration of all cancer cells is damaged.
- Otto Heinrich Warburg

Warburg articulated his hypothesis in a paper entitled “The Prime Cause and Prevention of Cancer”, which he presented in a lecture at the meeting of Nobel Laureates on June 30, 1966 (Lake Constance).

The significance of Dr. Warburg’s discovery is still apparent in the common cancer diagnostic test using fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET scan) which has a high diagnostic accuracy. The classic PET scan solution used is a variant of regular glucose which is tagged with a radioactive tracer so it can be detected by the PET scanner. Normal cells take up glucose at a relatively low basal rate. However, cancer cells, as previously described, rapidly use the glucose. These tagged glucose cells accumulate in the cancerous tissue and are seen as active sites of cancer growth.
PET Scan Detecting Lung Cancer
In his later years, Dr. Warburg became more convinced that most illness results from pollution or environmental toxins (xenobiotics). This led to a passionate focus on his health and diet. When he visited restaurants, he would bring his own tea bags with him. He also went to great lengths to obtain organic butter, the quality of which he trusted.
As early as 1923, Warburg and Schoeller discussed starving cancer by drugs leading to “nutritional deprivation”. In his last publication in 1970, (Entstehung von Krebsstoffwechsel durch Vitamin-B1-Mangel Thiaminmangel), Dr. Warburg claimed that a cause for spontaneous “tumor metabolism” was either a lack of oxygen or a lack of vitamin B1 (thiamin). He maintained that both conditions increase the production of lactic acid. This line of thinking led him to consider the administration of specific vitamin supplements, which would enhance cellular oxidation. Thus, he believed cancer could be prevented or treated nutritionally by maintaining an appropriate, organic nutrient-dense diet.
Dr. Warburg never married but had numerous friends and was accompanied faithfully by his longstanding companion Jakob Heiss. By most accounts, he never showed any interest in a typical social life. Like his father before him, Dr. Warburg resided in his Institute, working 6-day weeks on problems of cell physiology, particularly pertaining to metabolism, cancer and photosynthesis. He occasionally allowed only his love of riding horses to interrupt his research. In 1968, he suffered a broken femur. This was complicated by deep vein thrombosis. He died August 1, 1970 (at the age of 86) from a pulmonary embolism and was buried in a Christian cemetery.

Dr. Warburg was known to quote an aphorism he attributed to Max Planck:
Science advances one funeral at a time.
Dr. Otto Warburg was a pioneering biochemistry researcher who made substantial contributions to our early understanding of cancer metabolism. He was awarded the Nobel Prize in Physiology in 1931 for his discovery of the respiratory enzyme cytochrome c oxidase (CcO), which resides at the inner mitochondrial membrane.
Dr. Warburg demonstrated that cancers frequently rely less on mitochondria and obtain as much as 50% of their ATP by metabolizing glucose directly to lactic acid, even in the presence of oxygen. This frequent phenotype of cancers became known as the “Warburg Effect”.
Defects in mitochondrial oxidative phosphorylation complexes, altered bioenergetics and a metabolic shift to fermentation are often seen in cancers. Dr. Warburg’s research has now been proven to show that a defect in a mitochondrial electron transport component, the cytochrome c oxidase, leads to increased glycolysis and carcinogenesis.
Dr. Warburg was a role model for meticulous research, contributing so much towards our knowledge of how best to diagnose and treat cancer.
The metabolism of glucose, the central macronutrient, allows for energy to be harnessed in the form of adenosine triphosphate (ATP) through the oxidation of its carbon bonds. This process is essential for sustaining all mammalian life. In mammals, the end-product can be lactate or, upon full oxidation of glucose via respiration in the mitochondria, CO2. In tumors and other proliferating or developing cells, the rate of glucose uptake dramatically increases, and lactate is produced, even under fully oxygenated conditions. This process is now known as the Warburg Effect or briefly described as aerobic glycolysis.
Glycolysis is a metabolic pathway that occurs in the cell cytoplasm and involves a sequence of ten enzymatic reactions. These reactions convert glucose to pyruvate and produce the high-energy compounds ATP and nicotinamide adenine dinucleotide (NADH).
Dr. Warburg discovered that lactate production does not depend on the presence of oxygen. That had not been expected, since according to Pasteur, the presence of oxygen should have suppressed glycolysis. This is known as the Pasteur Effect (Pasteur’s observation that yeast cells consume less sugar when grown in the presence of O2 than when grown in the absence of it).
The fact that there appeared to be no direct relationship between respiration and glycolysis led to the conclusion that in cancer cells, glycolysis was a reaction which could produce energy, independent of respiration (oxygen consumption). In other experiments with varying glucose and bicarbonate concentrations, it was shown that there was no generalizable difference in oxygen consumption between the tumor and the respective normal epithelial tissue.
Dr. Warburg’s observation was that most cancer cells predominantly produce energy by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, rather than by a comparatively low rate of glycolysis followed by oxidation of pyruvate in mitochondria as in most normal cells. The latter process is aerobic (uses oxygen).
Malignant, rapidly growing tumor cells typically have glycolytic rates up to 200 times higher than those of their normal tissues of origin; this occurs even if oxygen is plentiful. In most non-cancerous cells, on the other hand, fermentation will not occur if there is enough oxygen.
The Warburg Effect

In the early 1970s, the Austrian biochemist Efraim Racker first coined aerobic glycolysis of cancer cells the Warburg Effect. Dr. Racker developed his own theories about the origins of the Warburg Effect ranging from imbalances in intracellular pH to defects in ATPase activity.
Essentially, what has come to be known as the Warburg Effect denotes a metabolic phenotype typical of many tumor cells, namely the high activity of aerobic glycolysis, i.e., production of lactic acid, even in the presence of sufficient oxygen. Dr. Warburg further attempted to expand the implications of this metabolic process to a general theory for the development of cancer.
For Dr. Warburg the onset of fermentation on the cellular level is the primary cause of cancer (“die letzte Ursache”). He suggested that cells turn into cancer cells by switching from respiration to fermentation and postulated this change in metabolism is a fundamental cause of cancer. Thus, instead of fully respiring in the presence of adequate oxygen, cancer cells ferment glucose. In short, cancer cells rewire their metabolism to promote growth, survival, proliferation, and long-term maintenance. The common feature of this altered metabolism is increased glucose uptake and fermentation of glucose to lactate.
The Warburg Effect is now a term used to describe two unrelated observations in oncology as well as plant physiology, both from the work of Dr. Warburg. In oncology, the Warburg Effect refers to the high rate of glycolysis and lactate fermentation in the cytosol exhibited by most cancer cells, relative to the comparatively low rate of glycolysis and oxidation of pyruvate in mitochondria exhibited by most normal cells. Thus, the Warburg Effect is the reverse of the Pasteur effect (the inhibition of fermentation by O2) exhibited by cancer cells. Alteration of the Pasteur effect in cancer is linked to prolyl hydroxylases and hypoxia-inducible factor. In plant physiology, the Warburg Effect is the inhibition of photosynthetic CO2 fixation by high concentrations of O2.
This discovery came to be a highly controversial issue in biochemistry climaxing in his famous paper in Science in 1956 - On the origin of cancer cells.

Over the past two decades, the discoveries of oncogenes and tumor suppressor genes have created a paradigm in which cell-autonomous genetic alterations were perceived as a primary driving force for neoplastic transformation. Oncogenic alterations of cell metabolism were considered as epiphenomena. However, with the discoveries of oncogenic mutations in mitochondrial metabolic enzymes, such as fumarate hydratase, succinate dehydrogenase, and isocitrate dehydrogenase 2, it is now unsound to deny the role of metabolism in tumorigenesis.
Dr. Warburg reasoned that respiration must be damaged in cancers because high levels of O2 are unable to suppress the production of lactic acid by cancer cells. Although the observations of Chance and Weinhouse in the 1950s initially negated Dr. Warburg’s contention of mitochondrial defects in cancers, many studies over the past several decades have documented oncogenic nuclear and mitochondrial DNA mutations in proteins involved in respiration. Thus, the Warburg Effect could arise from mitochondria DNA mutations and defective respiration. However, aerobic glycolysis can also occur concurrently with mitochondrial respiration. Viewed from the perspective of contemporary cell energetics, these newly transformed cancer cells possess an “inefficient" mechanism to produce ATP that favors aerobic glycolysis and lactate production in the cytosol instead of glucose oxidation progressing through mitochondrial oxidative phosphorylation.
The question remains as to why this occurs?
Mitochondrial Defects and the Warburg Effect (Yesterday and Today)
Possible Benefits of Aerobic Glycolysis on Cancer Cells
Increasing glycolysis may provide cancer cells with some advantages. The primary advantage or function is that some of this metabolic switch allows the pyruvate and lactate generated from glycolysis to go towards biosynthesis which is important for cancer cellular proliferation (growth). Lactate-producing cancer cells are characterized by increased aerobic glycolysis and excessive lactate formation.
In lactagenic cancers, oncogenes and tumor suppressor mutations behave in a highly orchestrated manner, apparently with the purpose of increasing glucose utilization for lactagenesis purposes and lactate exchange between, within and among cells.
Five main steps are identified:
-
increased glucose uptake;
-
increased glycolytic enzyme expression and activity;
-
decreased mitochondrial function;
-
increased lactate production, accumulation and release; and
-
upregulation of monocarboxylate transporters MTC1 and MCT4 for lactate exchange. Lactate is probably the only metabolic compound involved and necessary in all main sequela for carcinogenesis, specifically: angiogenesis, immune escape, cell migration, metastasis and self-sufficient metabolism. Additionally, excess lactate adds to an acidic extracellular terrain that may help cancer cells to survive and grow.

Research has even suggested that aerobic glycolysis may help cancer cells avoid being recognized and killed by cells of the immune system. Changes in the metabolic environment may block immune cells from finding cancer cells and even attract cells that may help tumor cells grow. The unusual metabolic changes seen in cancer cells may also activate oncogenes that allow cancer cells to avoid death.
Another proposed explanation as to why the tumor microenvironment selects for altered metabolism is that as the early tumor expands, it outgrows the diffusion limits of its local blood supply, leading to hypoxia (lack of oxygen) and stabilization of the hypoxia-inducible transcription factor (HIF).
HIF initiates a transcriptional program that provides multiple solutions to hypoxic stress. Because a decreased dependence on aerobic respiration becomes advantageous, cell metabolism is shifted toward glycolysis by the increased expression of glycolytic enzymes, glucose transporters, and inhibitors of mitochondrial metabolism. In addition, HIF stimulates angiogenesis (the formation of new blood vessels) by upregulating several factors, including most prominently vascular endothelial growth factor (VEGF). These new blood vessels feed the tumor with growth nutrients.
It should be emphasized that while malignant tumors have acquired a high glycolytic phenotype, enhancing their glucose uptake by almost 10-fold, their respiration rate remains essentially unchanged from that of surrounding normal tissue. Peter Pedersen and colleagues have further discovered select key sub-cellular and molecular players that propel the tumor toward this metabolic transformation. These being hexokinase, several mitochondrial associated enzymes and plasma membrane transporters, and the crucial organelle involved in energy generation, the mitochondrion.
While maintaining their capacity for respiration, tumors “turn more parasitic” by enhancing their ability to scavenge glucose from their surroundings. With excess glucose at hand, tumors shunt their metabolic flux more toward glycolysis than do their normal cells of origin, a strategy that allows for their survival when oxygen is limiting while providing them a mechanism to poison their extracellular environment with acid, thus paving the way for invasion and metastasis.
Significantly, tumors harness a crucial enzyme to regulate and support this destructive path and to entrap and channel glucose toward glycolysis. This enzyme is an isoform of hexokinase, referred to as hexokinase type II, or HK-2. HK2 is the first of several enzymes in cancer cells involved in metabolizing the sugar glucose to lactic acid. At its mitochondrial location HK2 binds at/near the protein VDAC (voltage dependent anion channel), escapes inhibition by its product glucose-6-phosphate, and gains access to mitochondrial produced ATP. It helps immortalize cancer cells, i.e., prevents their cell death.
Due to many-faceted molecular features at genetic, epigenetic, transcriptional, and enzymatic levels, including sub-cellular localization to mitochondria, HK-2 facilitates and promotes the high glycolytic tumor phenotype. Thus, HK-2 represents a pivotal model gene or enzyme that tumors “select for” during tumorigenesis in order to facilitate their destructive path.
Warburg Effect and Beyond: Encouraging Prospects for Effective Therapies
Recent studies have described how this aberrant glycolytic flux can be subverted toward a more “normal” metabolic phenotype, and how the glycolytic flux affects the tumor microenvironment to facilitate tumor metastasis. From this model several metabolic targeting strategies have been proposed that can selectively debilitate tumors. According to Dr. Pedersen and colleagues, an obvious first choice is to block the initiating steps of glycolysis so that it is prevented from advancing beyond the hexokinase mediated phosphorylation step.
An example of this is the inhibitor of hexokinase called 3-bromopyruvate (3-BP). Young Hee Ko and colleagues have successfully tested 3-BP in pre-clinical trials against malignant tumors and have shown it to be especially effective in eradicating advanced tumors in 19/19 animals, without harm to the animals, and without return of the tumors during their life. This research in the area of HK-2 inhibitors is beyond this biography but is proving to be a very promising cancer therapy.
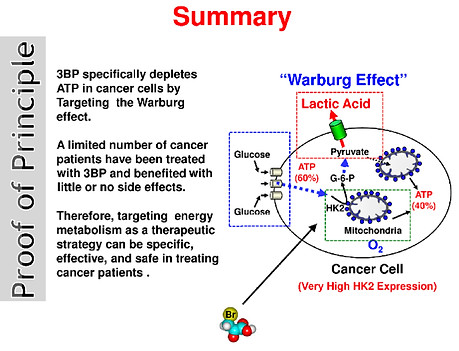

Another commonly used cancer treatment strategy based on controlling glycolysis and targeting the Warburg Effect is the ketogenic diet. This calls for minimizing carbohydrates and replacing them with healthy fats and moderate amounts of high-quality protein. The rationale in providing a fat-rich, low-carbohydrate diet in cancer therapy is to reduce circulating glucose levels and induce ketosis such that cancer cells are starved of energy while normal cells adapt their metabolism to use ketone bodies and survive. Furthermore, it also reduces blood glucose levels, which causes insulin and insulin-like growth factor (important drivers of cancer cell proliferation) to drop as well. Based on the results of rigorous preclinical and clinical studies performed thus far, the ketogenic diet appears to be a promising and powerful option for adjuvant therapy for a range of cancers.
Ultimately, the Warburg Effect is a fascinating area of cancer research that has been studied extensively since the 1920s with a surge in the number of publications from the 2000s to today. Its mechanism and role in cancer genesis and proliferation is gradually being understood. Its understanding holds great promise for finding a cure for many types of cancer.
The Ketogenic Diet
The physicist Manfred von Ardenne, who worked with Dr. Warburg, discovered that cancer cells, owing to their fermentation, are more acidic inside and on their surface, than normal cells. This has led to ideas of incorporating metabolic alkalization of the extracellular matrix to treat the cancer terrain. Additionally, the cancer cell’s acidity and altered metabolism makes it more sensitive to high temperatures. On this basis, whole-body hyperthermia and local hyperthermia has been incorporated clinically to treat tumors.
Here BRMI speaks about whole-body hyperthermia with the son of Manfred von Ardenne (who worked with Otto Warburg to develop a multi-step therapy to treat cancer).
Equally fascinating is the lifetime research of Peter Pedersen who describes via a series of sequential discoveries touching five decades how despite some impairment in the respiratory capacity of malignant tumors, that hexokinase 2 (HK-2), its mitochondrial receptor (VDAC), and the gene that encodes HK-2 (HK-2 gene) play the most pivotal and direct roles in the Warburg Effect.
They also discovered that like a “Trojan horse” the simple lactic acid analog 3-bromopyruvate selectively enters the cells of cancerous animal tumors that exhibit the Warburg Effect and quickly dissipates their energy (ATP) production factories (i.e., glycolysis and mitochondria) resulting in tumor destruction without harm to the animals.
Unfortunately, we still do not know all the “whys” or the “purpose” of the Warburg Effect, its role in cancer growth and carcinogenesis. Much of this is because attention has been biased towards gene-based research, chemotherapeutics and pharmaceutical “biologicals”.
While the Warburg Effect is a hallmark of cancer, the study of cancer cell metabolism was diverted when investigators began to employ genomic techniques to better understand cancer biology. Science has since regretted the historical lack of interest and understanding about the meaning and role of the Warburg Effect. It is unfortunate that metabolic research did not progress in parallel to gene-based research.
This tragic history has impeded the full comprehension of cancer biology, and, consequently, the development of effective therapeutic approaches based on an understanding of the roles of lactate in promoting carcinogenesis and tumorigenesis.
Although there have been important advances in the identification of oncogenes, tumor suppressor mutations and epigenetics, as well as some therapeutic applications, the cure for cancer through gene-based research has yet to come to fruition. Much of the research to date points to the conclusion that cancer may best be thought of as a metabolic disease rather than a genetic one.
Dr. Warburg’s Notable Works
-
Stoffwechsel der Tumoren (1926)
-
Katalytische Wirkungen der lebendigen Substanz (1928)
-
The Metabolism of Tumours (1931)
-
Schwermetalle als Wirkungsgruppen von Fermenten (1946)
-
Wasserstoffübertragende Fermente (1948)
-
Mechanism of Photosynthesis (1951)
-
Entstehung der Krebszellen (1955)
-
Weiterentwicklung der zellphysiologischen Methoden (1962)
-
The Prime Cause and Prevention of Cancer (1966)






